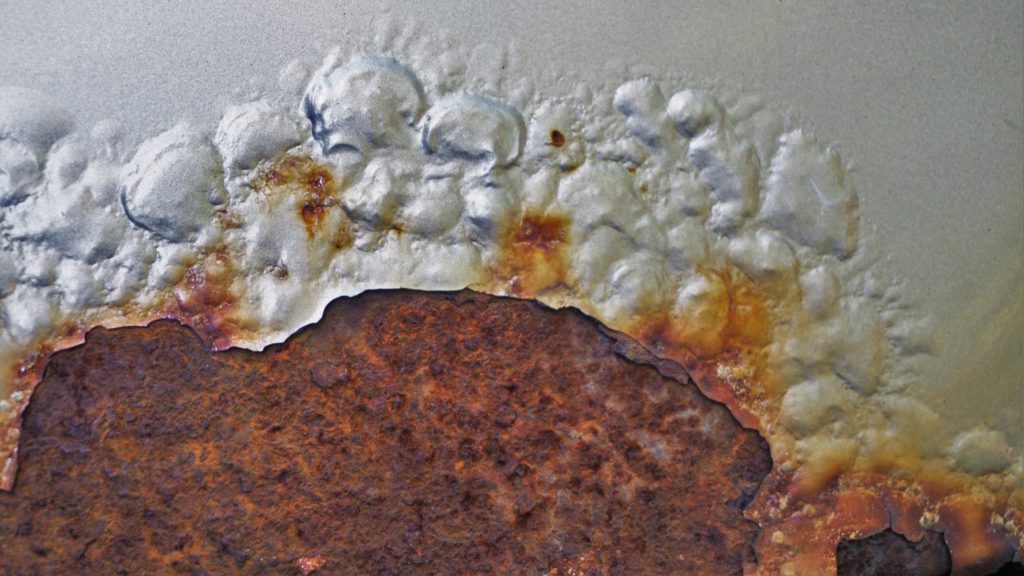
Blisters and bubbles occur all the time on industrial equipment. While it’s not always obvious why they form, it is important to be able to diagnose the source and avoid them in future situations. We want to give some insight so that you understand why a high-quality coating is so critical.
The first thing to note is that although often used interchangeably, blistering and bubbling are not the same. Generally, anything formed by a sort of osmosis is considered a blister, whereas anything outside of that would be considered a bubble. If you need to brush up on your terminology, osmosis describes the process of moisture molecules transferring through a semipermeable membrane (in this instance, the coating).
Osmotic blistering is the most common with carbon steel coatings because they often experience immersion service or excessive exposure to high-moisture environments. Combined with these qualities, the following factors almost guarantee a collection of moisture in the coating, causing osmotic blistering…
- Water-soluble salts…Chlorides, sulfates, and nitrates fall into this category. Since none of these are visible, it can be hard to identify them when applying a coating unless you are able to perform specific analytical testing.
- Water-soluble solvents…when these are trapped in the blisters, they can usually be recognized by their smell. However, analytical testing is still necessary.
These factors are even more likely to occur when there is a significant amount of moisture in contact with the coating. This is largely a result of immersion service. If there is any difference in the concentration levels of salts/solvents on either side of the coating, this can also be a factor as it creates osmotic pressure, eventually breaking through the coating itself. The moisture will naturally gather in places where the most concentrated portion of salts/solvents exists. Simply in an attempt to equalize pressure on both sides of the coating, this process is drawn out and eventually can cause a blister.
- Temperature differences across the coated surface…you may have heard this referred to as the “Cold Wall Effect”. When the temperature of the tank or vessel is lower than the temperature of what’s inside of it, blisters are formed. The warmer water molecules make their way into the coating film and condense at a cooler interface. This continues to happen until the pressure has to form into a blister in the coating. Exterior insulation is a good way to avoid these temperature differences and their consequences.
Now that you know way too much about blisters…let’s talk about bubbles. What’s the difference? We already mentioned it’s all in the way they form. Bubbles are formed from any NON-Osmotic events, which are typically environmentally charged…
– Applying a coating in direct sunlight
– Applying a coating too thick
– Applying a coating in humid weather
– Applying a coating in cool temperatures
All of these can cause an uneven drying process, leading to vapor pressure, eventually resulting in bubbles. Other scenarios, such as applying a coating over porous substrates or moisture could potentially cause bubbles as well.
A whole lot of information, we know. But overall, the important thing to know is how critical the environment in which you apply your coating is to the longevity and functionality of your equipment. Hopefully, this has made you aware of just how many critical elements are working together in this process. At Spear Industrial, we consider all of these and more when coating your equipment. Quality and safety are our top priorities, so let us handle the details. Call (432) 606-4093 to see how we can help with your next project.